猪群规模对猪群健康的影响(三)
来源:猪译馆 2020-09-21 15:22:37| 查看:次
译者的话<<
猪群规模对猪群健康的影响(三)
Empirical and theoretical evidence for herd size as a risk factor for swine diseases – Part 3
lan A. Gardner*, Preben Willeberg2,3 and Jan Mousing3+
1美国加利福尼亚戴维斯分校,兽医学院,医学与传染病学系
1Department of Medicine and Epidemiology, School of Veterinary Medicine, University of California, Davis, CA 95616, USA
2丹麦兽医及食品管理局
2Danish Veterinary and Food Administration, Mørkhøj Bygade 19, DK-2760 Soborg
3丹麦培根和肉类委员会
3Danish Bacon and Meat Council, Axelborg, Axeltorv 3, DK-1609 Copenhagen V, Denmark
猪群内和猪群间病原传播风险更大
Greater risk of transmission of infectious agents within and among herds<<
Koopman和Longini (1994)已经阐明影响个体间病原传播的因素。简而言之,这些因素包括受感染个体的传染力、通过不同传播途径散播病原体的数量和质量、单位时间内(针对不同途径)的接触次数、未感染者的易感性以及相互接触个体的数量。类似影响病原体在猪群间传播的因素包括:受感染猪群的传染力、未感染猪群的易感性、接触过程中散播活病原体的数量、接触频率以及发生接触的猪群数量(Stegeman等,1999)。
Factors that affect the transmission of infectious agents among individuals have been described by Koopman and Longini (1994). Briefly, these factors are the infectiousness of infected individuals, the quantity and quality of the agent transferred by the different routes of transmission, the number of contacts per unit time (for the different routes), the susceptibility of non-infected individuals, and the number of different individuals for which there is contact. Analogous factors affect the transmission of pathogens among herds: infectiousness of infected herds, the susceptibility of non-infected herds, the amount of viable pathogen that is transferred during a contact, the rate at which contacts occur and the number of herds that make contact (Stegeman et al., 1999).
为了解释和预测疾病发生模式,提出了许多疾病传播模型。在此,仅提及其中一个:Reed-Frost模型(Fox等,1971;Frost,1976; Yorke等,1979;Anderson和May,1982)。此模型能够预测有效接触率、易感猪只数量,以及感染猪对病原传播的影响:
To help explain and predict disease patterns, many models of disease transmission have been proposed. For brevity we mention only one: the Reed-Frost model (Fox et al., 1971; Frost, 1976; Yorke et al., 1979; Anderson and May, 1982). The Reed-Frost model predicts that the effective contact rate and the number of susceptible and infected pigs will affect the spread of an agent in a herd:
Ct+T = St(1-qCt) (4)
其中Ct指时间t内的感染数量,St是时间t内的易感猪只数量,q是指在持续时间T中,接下来一个时间段内易感猪只不被感染的概率。该公式表明疫病流行很大程度上取决于易感猪只数量和接触率之间的函数关系。若不通过疫苗接种降低感染风险,大规模猪群中易感猪只数量通常更多。此外,由于处于共同区域或空间,与易感和未免疫猪直接、间接接触的概率增加,有效接触的概率也可能更大。同一空间内饲养密度越大,猪之间的直接接触通常也会增加。在Reed-Frost模型中,这些因素的变化将导致不同时期呈现不同感染模式(如图2所示),也会影响在特定日龄或屠宰前的猪只总数(通过比较图2a和b可明显看出)。
where Ct is the number of cases at time t, St is the number of susceptible pigs at time t, and q is the probability that a susceptible pig will not become infected during the next time period of duration T. This equation indicates that the occurrence of an epidemic is largely a function of the number of susceptible pigs and the contact rate. The number of susceptible pigs is usually greater in large herds unless their risk of infection is reduced by vaccination. Moreover, the probability of an effective contact might also be greater because of housing in common areas or air spaces, and the increased likelihood of direct and indirect contact with susceptible, non-immune pigs. Direct contacts between pigs in the same space often increases with increasing stocking density. In the Reed-Frost model, changes in these factors will result in different patterns of incident cases with time (Fig. 2) and in the total number of affected pigs before a given age or at slaughter (as is evident by comparing Fig. 2a and b).
尽管公式4中q可能与猪群规模无关,但猪群规模效应显示出,模型中应包含猪群规模(用N表示)。因此,De Jong(1995)所提出公式中猪群规模可决定传播率(SI/N。其中S是易感猪只数量,I是感染猪只数量,N是猪只总数;详见 De Jong,1995年):
Although q in equation 4 could be independent of population or herd size, a herd-size effect implies that herd size (denoted N) should be incorporated explicitly in the model. Accordingly, De Jong (1995) has recommended the following form with a herd size-dependent transmission rate (SI/N. where S is the number of susceptible animals, I is the number of infectious animals and N is the total number of animals; for a more detailed discussion see De Jong, 1995):
Ct+T = St(1-e-βCtT/N) (5)
由于宿主、病原和环境因素之间的相互关系,以及所涉及的病因复杂,且育肥猪研究中针对结果的衡量标准主要采用患病率而不是发病率,所以对于肺炎和胸膜炎,就个体而言,实验数据很难直接与Reed-Frost模型的理论预测进行比较。育肥猪肺炎和胸膜病变的患病率取决于生长期/育肥期的病变发生率,以及感染时日龄的分布和病变治愈率。有证据表明,肺部病变在屠宰前可部分或全部治愈;慢性胸膜炎病变治愈率有限(Christensen和Mousing,1992)。
For pneumonia and pleuritis, direct comparison of empirical data with the theoretical predictions of the Reed-Frost model at an individual animal level is difficult because of complex interrelationships among host, agent and environment factors, the involvement of multiple etiological agents, and because prevalence rather than incidence is the usual outcome measure in studies of slaughter pigs. The prevalence of pneumonic and pleuritic lesions in slaughter pigs determined by the incidence rate of lesions during the grower/finisher phase, the distribution of age at infection and the rate of lesion resolution. There is evidence that many pneumonic lesions heal partly or completely before slaughter; resolution of chronic pleuritis lesions is more limited (Christensen and Mousing, 1992).
PRV在猪群内传播,因为只涉及单一病原,且为慢性感染(至少血清学检测呈阳性),所以对猪群规模效应评估更为容易。Duffy等(1991a)提出,大型猪群的后备母猪引种数量多于小型猪群;假定后备母猪在引种时为PRV阴性,那么大型猪群中的易感母猪会多于小型猪群。一项PRV确定性数学模型也表明,病毒持续感染的决定因素是猪群规模和母猪饲养密度。已确定母猪群规模的临界值为66头,低于临界值,即使不采取任何控制措施,病毒也可清除(Smith和Grenfell,1990)。但是,Bouma等人(1995),在10头和40头已免疫接种的猪群中运用易感 - 感染 - 恢复(SIR)模型,未能证明PRV传播与猪群规模有关。
For PRV evaluation of the effects of herd size on within- herd transmission is easier because a single infectious agent is involved and pigs remain chronically infected (or at least serologically positive for life). Duffy et al. (1991a) proposed that more replacement gilts are introduced in large breeding herds than in small herds; therefore, large herds will usually have more susceptible females than small herds—provided that replacement gilts are PRV-negative at introduction. A deterministic mathematical model of PRV showed also that the most important determinants of viral persistence were herd size and the density at which sows were maintained. A threshold herd size of 66 sows was identified, below which virus would be eliminated from the herd even when no specific control measures were implemented (Smith and Grenfell, 1990). In contrast, Bouma et al. (1995), using a susceptible-infectious-recovered (SIR) model, failed to show that the PRV transmission in groups of 10 and 40 vaccinated pigs was dependent on population size.
猪群规模相关的管理因素影响
Influence of management-related factors that are associated with herd size<<
在猪群中,管理和环境因素是相互关联的,且通常与猪群规模有关。大型猪群与小型猪群不只是猪只数量或饲养密度的区别。因此,猪群规模效应(正相关或负相关)经常与其他因素的影响混淆,这些因素本身可能没有记录,但确实与猪群规模有关。这些因素因猪群和国家而异,有些在大规模猪群中更常见,如封闭式圈养(Anderson等人,1990)、全进全出和多点式生产系统、自动化及工人雇佣,但其他情况,如购买小猪用以育肥,这在一些国家的小规模猪群中更常见(Pointon等,1985)。专业化大型猪群更注重进行新圈舍内的环境提升,如建立小隔间(Elbers, 1991),但是最近以动物数量为基础,描述不同猪群规模或不同日龄圈养猪只的相对活动频率的研究很少。
In swine herds, management and environmental factors are interrelated and often associated with herd size. Large herds are different from small herds in many ways other than merely the number of pigs or stocking density. Thus, any herd-size effect (positive or negative) is often mixed with the effects of other factors which may not be recorded per se but which are associated with herd size. These factors vary from herd to herd and from country to country, but many factors, including confinement housing (Anderson et al., 1990), all-in, all-out and multisite production systems, automation and the use of hired workers, probably occur more frequently in large herds, while others, such as the purchase of pigs for finishing, are more frequent in small herds in some countries (Pointon et al., 1985). Specialized large herds often incorporate improved environmental features in new buildings, including small compartments (Elbers, 1991), but there are few recent population-based studies which have described the relative frequency of practices in herds of different size or in herds with buildings of different ages.
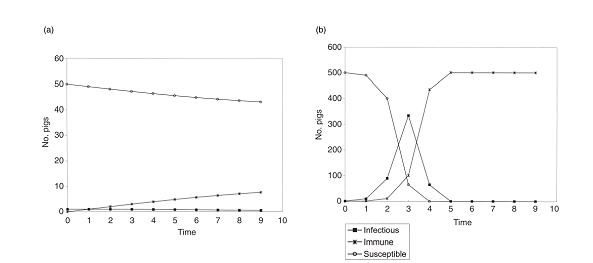
图2. 初始猪群规模为50头(a)和500头(b),接触率为0.02,使用Reed-Frost模型预测动物的感染患病数量。“免疫”这一类别包含有长期感染风险的猪。
Fig. 2. Reed-Frost model estimates of the number of cases of an infectious disease for a contact rate = 0.02 and for initial population sizes of 50 (a) and 500 susceptible pigs (b). The category ‘immune’ includes pigs at risk of being chronically affected.
疾病控制措施,如疫苗接种和用药程序等,也会因猪群规模而异(Svensmark等,1989a,b;Siegel和Weigel,1999)。在美国,猪群中母猪数量增加,会更多采用疫苗来控制繁殖及新生动物疾病(美国农业部;动植物检疫局;兽医处,1995)。如果大型猪群比小型猪群使用疫苗更频繁,且接种有效,那么大型猪群中免疫接种可降低病原传播风险。虽然接种疫苗可以改变未感染猪的易感性,也可以影响感染猪的病原排散,但疫苗并非总是对所有疾病有效。例如,胸膜肺炎放线杆菌等病原体的疫苗常常无法起到保护作用(Hunneman,1986)。
Disease-control practices such as vaccination and medication policies are also likely to differ by herd size (Svensmark et al., 1989a, b; Siegel and Weigel, 1999). In the USA, for example, the use of vaccines to control reproductive and neonatal diseases increases with increasing number of breeding females in the herd (United States Department of Agriculture; Animal and Plant Inspection Service; Veterinary Services, 1995). If large herds use vaccine more frequently than small herds and vaccination is effective, then vaccination might counteract any increased risk of transmission in large herds. Although vaccination can alter both the susceptibility of non-infected pigs and the shedding of pathogens by infected pigs, the assumption that vaccines for all diseases are effective is inappropriate. For example, vaccines for agents such as Actinobacillus pleuropneumoniae have often failed to be protective (Hunneman, 1986).
人们在养猪方面的技能和经验会对新生仔猪死亡率、繁殖性能和其他生产力指标产生影响(Wilson等,1986)。尚未证实这些技能和经验是否对传染病也有影响,但聪明的饲养员或管理者也许能够及早发现并进行临床干预,从而影响疾病进程。常表现于屠宰时病变的出现频率降低以及病变严重程度的降低。很难用客观的方式量化饲养员或管理者的知识、技能,因此,通常将此类影响列入其它的猪群效应中。
The skill and experience of people interacting with the pigs can influence neonatal mortality, reproductive performance and other productivity measures (Wilson et al., 1986). Whether there is also an influence on infectious disease has not been demonstrated clearly but the astute herdsperson or manager may be capable of early detection and intervention in clinical illness and potentially influence the course of disease. Such an influence might be reflected in a decreased frequency and severity of lesions at slaughter. The skill and knowledge of the herdsperson or manager is especially difficult to quantify in an objective manner and, hence, this effect usually will be included with any residual herd effect.
在大型猪群中,一些重要的管理变量能够影响疾病发生,这些变量范围极大。Willeberg(1979)以及Martinsson和Lundeheim(1988)指出,在屠宰时发现胸膜炎病变的几率随每年屠宰猪数量的增加而增加,但是有些大型猪群的患病率与小型猪群相似。大型猪群间的这些差异,尚无解释,但可能与管理有关。
Substantial scope probably exists to identify important management variables that influence disease occurrence in large herds. Willeberg (1979) and Martinsson and Lundeheim (1988) showed that for pleuritis lesions at slaughter, prevalence increased with the number of pigs slaughtered per year, but there were some large herds that had prevalences similar to those in small herds. Explanations for these differences among large herds were not determined but were probably related to management.
在疾病的检测与淘汰过程中,即使净化过程中未发生感染扩散,大型猪群的清除感染时间(以隔离时间为准)也会变得更长。这可能归因于管理和圈舍因素、较高的初始感染率,或是因为使用敏感性低于100%的检测(在下一部分进行讨论)。例如,Siegel等(1993)研究了美国伊利诺伊州自愿进行猪群伪狂犬病净化时相关的隔离检疫因素。较大的猪群(大于80头母猪与30-80头母猪进行对比)隔离期更长。但是,猪群规模大、初始血清阳性率、隔离期以及推迟清群计划均正相关。将所有因素纳入考克斯(Cox)比例风险模型后,这些因素与大型猪群规模的正相关性降低,但相关性仍略显著。
During test-and-removal programs for diseases, time to eradicate infection (approximated by time under quarantine) could be greater for large herds even if no spread of infection occurs during the eradication process. This might be attributable to management and housing factors, a higher initial prevalence of infection, or to the use of tests with a sensitivity of less than 100%(discussed in the following section). For example, Siegel et al. (1993) studied factors associated with time under quarantine for swine herds in the voluntary phase of pseudorabies eradication in Illinois, USA. Larger herds (>80 versus 30-80 sows) had an increased quarantine time; however, large herd size, initial seroprevalence, confinement housing and delay in initiating a herd clean-up plan were positively interrelated. After inclusion of all factors in a Cox proportional hazards model, the positive association with larger herd size was reduced but was still marginally significant。
不完善的诊断检测与采样策略对猪群规模效应的影响
Herd size effects attributable to imperfect diagnostic tests and sampling strategies<<
清除感染的时间
Time to eradicate infection
进行疾病净化时,若检测方式敏感性不佳,则呈假阴性结果的预期数量与猪群规模成正比(假设已对整群进行检测,且猪群之间的检测敏感性和患病率恒定不变)(Martin等,1992)。假定某猪群猪的数量为n,检测敏感性为s,检测到猪群患病率为p,则预期被感染的猪,其被检测为阴性的数量用E表示,符合:E = np(1-s) (6)
When disease-eradication programs are based on tests of imperfect sensitivity, the expected number of false-negative test results is directly proportional to herd size (assuming that the entire population is tested and that test sensitivity and prevalence are constant from herd to herd) (Martin et al., 1992). Assuming that a herd with n pigs with a prevalence of infection p is tested with a test of sensitivity s, the expected number E of infected pigs that will test negative is
E = np(1-s) (6)
例如,检测患病率为10%的100头猪,敏感性为90%,产生1个假阴性结果,而检测具有相同患病率的1000头猪,以90%的敏感性检测则产生10个假阴性结果。通常,在受感染的猪群中会对首次检测呈阴性的猪进行再次检测,直至记录了预定数量的猪群阴性检测,从而提供足够的证据证明该猪群没有感染。因此,为清除感染而进行测试的轮次取决于猪群规模、首次出现假阴性结果与再次检测结果之间的相关性,以及病原在感染猪与易感猪之间的传播率。以上内容也适用于组合检测或合并检测(Christensen和Gardner,2000)。Siegel等人研究显示(1993),164个感染PRV的猪群中,只有9个采用了检测并清除的方法,因此无法确定这一理论考量是否真正适用于此研究。
For example, a test of 90% sensitivity used in a herd of 100 pigs with a prevalence of infection of 10% would be expected to yield one false-negative test result, while in a herd of 1000 pigs with the same prevalence it would yield 10 false-negative results. Usually, pigs testing negative on the first test in an infected herd are retested again until a predetermined number of negative herd tests are recorded that provide sufficient evidence that the herd is free of infection. The number of rounds of testing to eliminate infection should therefore depend on herd size, the correlation of the test-retest results of pigs that initially tested negative yet were truly infected, and the transmission rate of the pathogen between infectious and susceptible pigs. Similar considerations apply when combinations of tests or pooled tests are used (Christensen and Gardner, 2000). In the study by Siegel et al. (1993), only nine of 164 PRV-infected herds were on a test-and-removal program, so we are unable to determine whether this theoretical consideration truly applied to the study.
确定是否对猪群进行干预
Identification of the herd for intervention
另一方面,由于猪群敏感性(感染猪群检测呈阳性的概率,通常是指该群中至少有一头猪检测呈阳性)很大程度上取决于样本量,假如对猪群内固定比例猪只进行检测(而非对猪群内固定数量猪只),则更容易确定是否对较大的感染群体进行干预(假定其他影响猪群敏感性的因素保持不变)(Martin等,1992)。
On the other hand, because herd-level sensitivity (the probability that an infected herd will test positive, usually meaning that at least one pig in the herd tests positive) is strongly determined by sample size, a larger infected herd would be more likely to be identified for an intervention if a fixed proportion of the herd (rather than a fixed number per herd) were tested (assuming other determinants of herd-level sensitivity were held constant) (Martin et al., 1992).
服务热线:400-808-6188
Copyright©2010-2022 https://www.zhuwang.cc